Projects
DepMYC (2024->)
DepMYC is a translational, pre-clinical cancer drug discovery initiative that aims to develop MYC pathway dependent, molecular-targeted pan-cancer therapies for the treatment of advanced and drug-resistant cancers. DepMYC project allows for flexible design of combination treatments, including MYC pathway-directed drugs and optimized immunotherapies for effective, durable and safe therapeutics across diverse cancer types.
DepMYC explores synthetic lethal space around mitochondrial metabolism with a drug discovery program that broadly benefits from the synergy and resources available at the University of Helsinki, Finland and UCSF, USA. The program strives to replace generally toxic existing therapies with cancer cell specific, yet broadly applicable (for multi-cancer indications) and safe drugs to improve the quality of life of cancer patients without compromising treatment efficacy.
Funding: applied
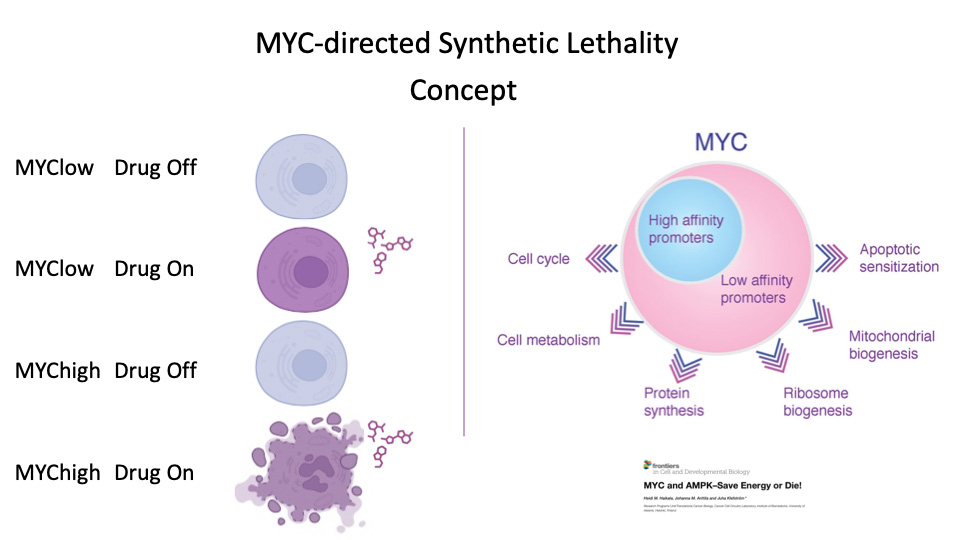
MYCimmune
MYC Cancer Gene and Immunity
MYCimmune is an international cancer research and innovation collaborative between Goga Lab UCSF (USA) and Klefström Lab (University of Helsinki, Finland), which strives to advance clinically viable MYC oncogene-directed therapeutic concepts to early phase clinical testing. The joint groups at the University of Helsinki and UCSF have discovered that the MYC oncogene is associated with poor breast cancer patient outcomes and resistance to immunotherapies. The project focuses on finding new improved treatments that selectively kill high-MYC tumor cells and improve response to immune treatments.
Funding:
- Combining Immunotherapy and MYC Synthetic Lethality for Metastatic Breast Cancer is provided by the US Department of Defense Breast Cancer Research Program (BCRP), Breakthrough Award W81XWH2110773
- MYC-based synthetic lethal strategies to treat metastatic breast cancer (project number 350393) by the Academy of Finland.
Publications
ReCurE (2023-2024)
ReCurE team is developing novel, gentler therapies based on the MYC gene, the driving force behind many cancers that cause solid tumors. Combined with immunotherapy, the new drugs could produce the same treatment outcomes without the side effects associated with cytostatic agents. ReCurE also examines models based on a living cancer tissue sample that provide novel platforms for testing drugs.
ReCurE looks for clinical translation and commercially viable options to develop novel cancer drug candidates and drug testing platforms. ReCurE is hosted by Klefström Lab. ReCurE team is led by Juha Klefström, PhD and Margareta Klabbers, LL.M, and consist of full-time medicinal chemist, assay developer and in vivo biologist.
Funding: ReCurE is a Business Finland funded R2B-project 7443/31/2022


RESCUER (2020-2025)
The RESCUER (RESistance Under Combinatorial Treatment in ER+ and ER- Breast Cancer) consortium gathers expertise and facilities to solve the challenge of identifying novel characterization methods for breast cancer drug resistance and new knowledge on effective combinatorial treatments. RESCUER consists of fifteen organizations from Belgium, Finland, France, Germany, Israel, Norway, Spain, Sweden, United Kingdom, and United States.
Funding: RESCUER has received funding from the European Union’s Horizon 2020 research and innovation programme, under grant agreement No. 847912.
Cancer IO (2020-2023)
Cancer IO is a cancer immunotherapy focused public-private collaborative for better research, innovation, health care and public awareness. With a total funding of 10 million euros, Cancer IO is one of the largest IO focused research and innovation programs in Europe.
Funding: Cancer IO is funded through Business Finland’s Personalized Health Program. The program ended in 2023.

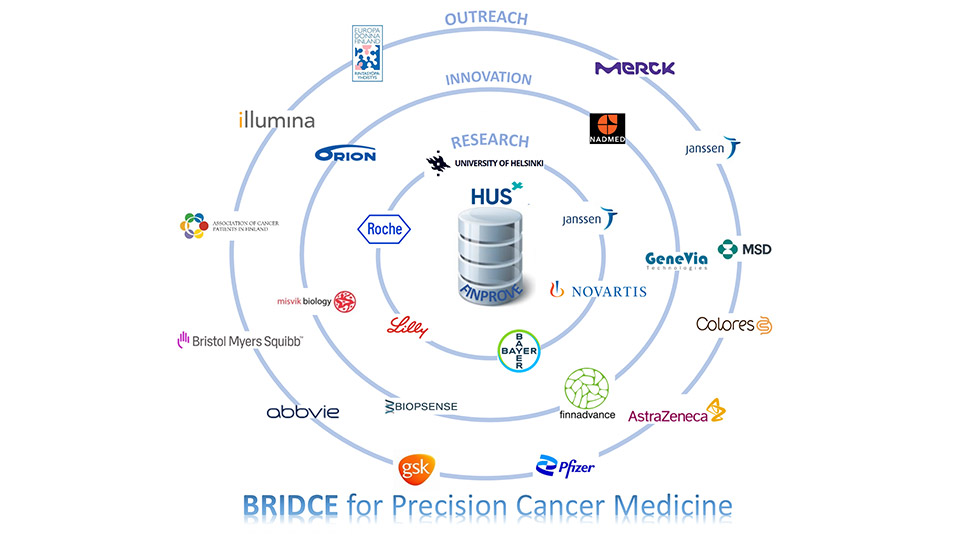
Bridce
The BRIDCE project is developed around the ambitious precision cancer research project FINPROVE run by Helsinki University Hospital. The aim of FINPROVE is to match the personal genomic data of cancer patients to medicines which are not yet available to use in clinical practise in Finland. This may create new opportunities for cancer patients to benefit from treatment. Built on this, BRIDCE enables, through patient consent, using the health data generated in FINPROVE to research and development activities of Finnish small companies. This may lead, for instance, to improved accuracy of cancer diagnostics. Through this, BRIDCE creates a new precision cancer ecosystem in Finland linking all relevant actors – public and private, researchers and companies – under a jointly agreed mission to improve cancer care in Finland.